Co-Diagnostic’s Revenue By Region (2019-2023)
Exclusive Data
You need the Pro Plan to access KPI data
- Full access to the platform
- KPI data & segment financials on US stocks
- Financial data on thousands of stocks
- Download data in xlsx and csv formats
Pro Plan
$49 per month*
60% discount ends in:
.
About
More information
Subscribe to Pro or Enterprise plans to unlock this feature.
Contact the Analyst
Subscribe to Pro or Enterprise plans to unlock this feature.
Become a smarter investor today.
Access KPIs & Segment Financials on US stocks
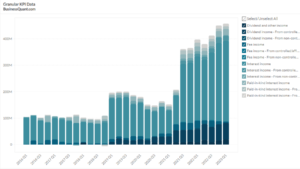
This statistic highlights Co-diagnostic’s Revenue by Region, split between the United States, and the Rest of the World, reported on a quarterly basis from Q1 2019 onwards.
The Co-Diagnostic company’s era has wide molecular programs: multiplexing, single nucleotide polymorphism (SNP) and mutation detection, real-time or conventional PCR detection, genotyping, RNA expression, and Sanger or next-generation sequencing. The variety of programs opens doorways for them to increase assessments associated with multiple disciplines: infectious sickness, oncology, transfusion medicine, pharmacogenetics, therapeutics, liquid biopsy, agriculture, or veterinary medicine. The company diagnostics structures permit very rapid, low-value, molecular checking out for organisms and genetic sicknesses with the aid of using automating, traditionally complicated, strategies for the improvement and management of assessments.
Co-diagnostic’s Revenue by Region
| Revenue by Region | Q3 2020 | Q2 2021 | Q3 2021 | Revenue Mix by Region in Q3 2021 |
| US | $14.05 | $5.37 | $16.91 | 56.18% |
| Rest of world | $7.76 | $21.99 | $13.19 | 43.82% |
| Total | $21.82 | $27.36 | $30.10 | 100.00% |
(All figures are in millions, except percentages)
The total revenue of Co-diagnostics has been on the rise. It rose from $21.82 million in Q3 2020 to $30.10 million in Q3 2021 indicating an increase of 38.03% on a year-on-year basis. It witnessed an increment of 10.01% on a quarterly basis, as compared to $27.36 million in Q2 2021. A major portion of the company’s revenue, accounting for about 56.18% of the total revenue in Q3 2021, is generated by the United States.
United States
Under their EUA, they’re actively promoting their LogixSmart COVID-19 check to CLIA licensed laboratories inside the United States. The CLIA labs qualify their LogixSmart check as a Laboratory Developed Test (LDT), a diagnostic check that has been verified to be used with inside the CLIA lab.
The majority of the total revenue of the company is generated by the United States, contributing about 56.18% in Q3 2021. It had shown an increase of $2.86 million, from $14.05 million in Q3 2020 to $16.91 million in Q3 2021, making an increase of 20.35% on a year-on-year basis. From Q3 2020 to Q2 2021, the revenue witnessed a huge drop of 61.78%. However, it again increased by 214.9% on a quarter-on-quarter basis, as compared to $5.37 million in Q2 2021.
Rest of the world
Molecular diagnostics, along with their assessments, are ruled in Europe with the aid of using the framework for in vitro diagnostics (IVDs), which encompasses diagnostic merchandise along with reagents, instruments, and structures supposed to be used inside the prognosis of sickness. The company has started a reagent apartment application in India with thermocyclers bought from third-birthday celebration providers. The placement of thermocyclers in India has facilitated the sale of the SaraGene COVID-19 assessments in India, which has made Sara Profitable in 2020.
Revenue from Other Countries of the world has a contribution of 43.82% in the total revenue of the company in Q3 2021. It has a revenue of $13.19 million in Q3 2021. This region had its maximum revenue in Q2 2021, a period where the US generated the least revenue. It increased by 69.9% on a year-on-year basis, from $7.76 million in Q3 2020 to $13.19 million in Q3 2021. However, it declined by 40.01% on a quarterly basis, as compared to $21.99 million in Q2 2021.
About Co-diagnostics Inc
Co-Diagnostics Inc., an Utah enterprise, is growing sturdy and modern molecular equipment for the detection of infectious sicknesses, liquid biopsy for most cancers screening, and agricultural programs. They increase, manufacture, and promote reagents used for diagnostic assessments that feature the detection and/or evaluation of nucleic acid molecules (DNA or RNA). They also promote diagnostic systems from different producers as self-contained lab structures. These are included in the sale of their assessments.
The company is a molecular diagnostics agency with a unique, patented Polymerase Chain Reaction (“PCR”) checking out era. The company commercialises their modern patented and patent-pending era through sales, improvement, and licensing, to rapidly increase molecular diagnostics which might be greater value-green and carry out higher than conventional technology of the past. The Company’s proprietary molecular diagnostics era is paving the manner for innovation in sickness detection and existing sciences studies through their more suitable detection of genetic material. They own their platform due to which they can accomplish this quicker and greater economically, making an allowance for wider margins even as nevertheless positioning Co-Diagnostics to be the worldwide low-cost leader of molecular diagnostics services. The common stock of the company is publicly traded on the NASDAQ under the symbol “CODX”.
Did you like Co-diagnostic’s Revenue by Region statistic?
Access more such KPI data points and segment financials on thousands of US stocks, with Business Quant.
You can get started here.
More data on US Stocks

Our Plans
Always know what you’ll pay. No hidden costs or surprises.
- Annual
- Monthly
60% discount till April 30
Pro
For serious investing
-
Company KPI data Access segment financials, non-GAAP metrics and KPI data from presentations and filings. Examples include financials by segment / region / product category, AT&T's broadband subscriber trends, Tesla's deliveries by model and lots more.
-
Stock research tools Features include : stock screener, stock comparison, industry financials, stock warnings, advanced charting tools, timeseries tables, scatter charts, financial statements, stock reports, SEC filings, stock ratings, institutional and insider ownership data. There are 200+ financial items and ratios on thousands of US stocks.
-
Industry data & tools Access premium operating data on 40+ industries. Examples include market share, smartphone shipments by vendor, subscribers by wireless carrier, historical gold production. There are 20,000+ such statistics.
Enterprise
For tailored workflows
-
All of Pro plan Get unfettered access to all our dashboards and dossiers.
-
Custom built features Get tailored dashboards built specially for you , based on your set of requirements, to simplify your research workflow.
-
Admin billing Back-end documentation support and multi-seat licensing.
* Billed annually, local taxes extra.
60% discount on Annual plan
Pro
For serious investing
-
Company KPI data Access segment financials, non-GAAP metrics and KPI data from presentations and filings. Examples include financials by segment / region / product category, AT&T's broadband subscriber trends, Tesla's deliveries by model and lots more.
-
Stock research tools Features include : stock screener, stock comparison, industry financials, stock warnings, advanced charting tools, timeseries tables, scatter charts, financial statements, stock reports, SEC filings, stock ratings, institutional and insider ownership data. There are 200+ financial items and ratios on thousands of US stocks.
-
Industry data & tools Access premium operating data on 40+ industries. Examples include market share, smartphone shipments by vendor, subscribers by wireless carrier, historical gold production. There are 20,000+ such statistics.
Enterprise
For tailored workflows
-
All of Pro plan Get unfettered access to all our features.
-
Custom built features Get tailored dashboards built specially for you , based on your set of requirements, to simplify your research workflow.
-
Admin billing Back-end documentation support and multi-seat licensing.
* Local taxes extra.